Innovative Chemists: Pioneers Who Battled Global Hunger
Written on
Chapter 1: The Prelude to Crisis
At the start of the 20th century, humanity was on the brink of disaster. A looming threat of widespread famine, anticipated due to a rapidly growing population, was about to manifest. In response, scientists worldwide were urged to find viable solutions. — Thomas Hager, The Alchemy of Air
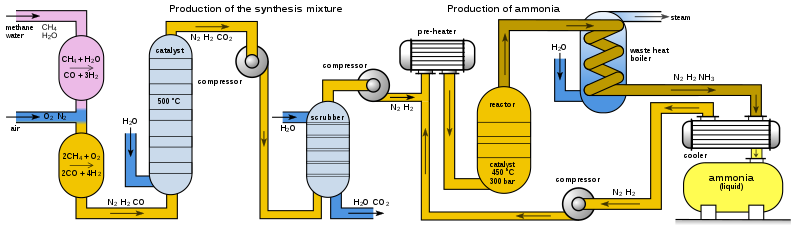
The German phrase “Bros aus Luft,” translating to “bread from air,” refers to a remarkable chemical innovation known as the Haber-Bosch process. According to Tim Harford from the BBC, this process is so pivotal that it’s estimated half of the global population owes its existence to it. The Haber-Bosch process is a method for converting atmospheric nitrogen into ammonia, which is fundamental for fertilizer production. This process is a type of nitrogen fixation.
In essence, the process involves using atmospheric nitrogen and hydrogen as a catalyst to produce ammonia. Achieving maximum ammonia output requires high pressure (around 200 atmospheres) and elevated temperatures (approximately 400-450°C).
For many chemists, these conditions posed significant safety risks. The renowned chemist Henry Le Chatelier experienced a catastrophic explosion in his lab while attempting to combine hydrogen and nitrogen under extreme conditions, nearly resulting in the injury of an assistant.
This left a gap for chemists to innovate new methods of nitrogen fixation. The German chemist Fritz Haber would rise to the challenge.
Chapter 2: The Innovation of Haber
In the early 1900s, Haber found himself in a competitive relationship with fellow chemist Walter Nernst, making nitrogen fixation his primary focus. Drawing on some of Nernst's findings and his own expertise in high-pressure chemistry, he devised a new ammonia production system.
Haber's system necessitated high pressure, one or more catalysts to enhance ammonia synthesis, elevated temperatures for efficiency, and a method for separating ammonia from other compounds while recycling heat.
Learning from Le Chatelier's accident and other high-pressure mishaps, Haber needed to explore equipment that could facilitate successful experiments. He tested various catalysts, including osmium and uranium, in addition to iron, nickel, and calcium.
Ultimately, Haber discovered that since the reaction was exothermic, he could harness the heat generated in the process. By 1909, he demonstrated that the method could produce sufficient ammonia to consider industrial-scale application.
Chapter 3: Industrialization of the Haber Process
The German chemical company BASF recognized the potential of Haber's process and sought to patent it. Initially, some researchers within the company were skeptical, believing that no industrial equipment at the time could sustain such extreme pressure and temperature safely and efficiently. The use of osmium raised additional concerns due to its limited availability.
However, influential scientists persuaded BASF President Heinrich Von Brunck to engage with Haber. Accompanied by several colleagues, they visited Haber's lab to evaluate the feasibility of industrializing the process. Upon learning about the required pressures, Brunck consulted Carl Bosch, a chemist with expertise in nitrogen fixation. Bosch confidently asserted, “I believe it can work. I know precisely what the steel industry can achieve. We should take the risk.”
With the collaboration of Haber, Bosch, and other researchers, the Haber process began its transition to industrial application. By 1910, Bosch successfully scaled the process for large-scale production, leading to the first ammonia manufacturing at the Oppau plant in Germany in 1913. This facility could produce one ton of liquid ammonia every five hours, generating 20 tons of nitrogen daily.
Haber and Bosch were awarded the Nobel Prize in Chemistry in 1918 and 1931, respectively.
Chapter 4: A Dual Legacy
Ammonia is crucial for creating fertilizers like ammonium nitrate. However, ammonium nitrate can also serve as an explosive, particularly when combined with fuel oil. During World War I, the Oppau plant rapidly shifted from fertilizer production to creating explosives for trench warfare, driven by the needs of the conflict.
Haber became increasingly involved in chemical warfare innovations during this period. According to historian Dietrich Stoltzenberg, he played a significant role in developing chlorine gas for use in combat, along with protective gas masks.
Despite his contributions to warfare, Haber faced criticism for his role in chemical weaponry. In the early 1930s, as a Jewish scientist, he grew increasingly alarmed by the rise of the Nazi party in Germany and its targeting of Jewish scientists. Despite his service to the state and conversion to Christianity, he faced hostility and was ordered to dismiss his German colleagues.
His family relocated to the United Kingdom in 1933, and his children became British citizens. He passed away the following year from a heart attack.
Bosch, after the success of the Haber-Bosch process, focused on synthetic fuel production and founded IG Farben, serving as its first head. Discontent with the rise of Hitler and the Nazi regime, he was eventually stripped of his positions and fell into depression and alcoholism, dying in 1940 in Heidelberg.
Conclusion: A Complex Legacy
Thomas Hager reflects, “Their monumental success came at a significant cost, one that we continue to bear. The Haber-Bosch process was also instrumental in producing the explosives that caused countless deaths during the world wars. Both men faced scorn during their lives, and both died in a state of disillusionment and disgrace.”
Haber and Bosch's scientific brilliance provided a means to avert mass starvation, a feat for which they are often unrecognized. However, their contributions also facilitated the development of chemical weapons and explosives responsible for immense loss of life during history's deadliest conflicts. Their legacies are intricate and multifaceted, yet one truth stands out: they deserve greater acknowledgment for their profound impact on humanity.